This diabetes technology offers a continuous stream of data to show a more complete picture of your blood sugars over time. This is different from a fingerstick meter that only represents that moment.
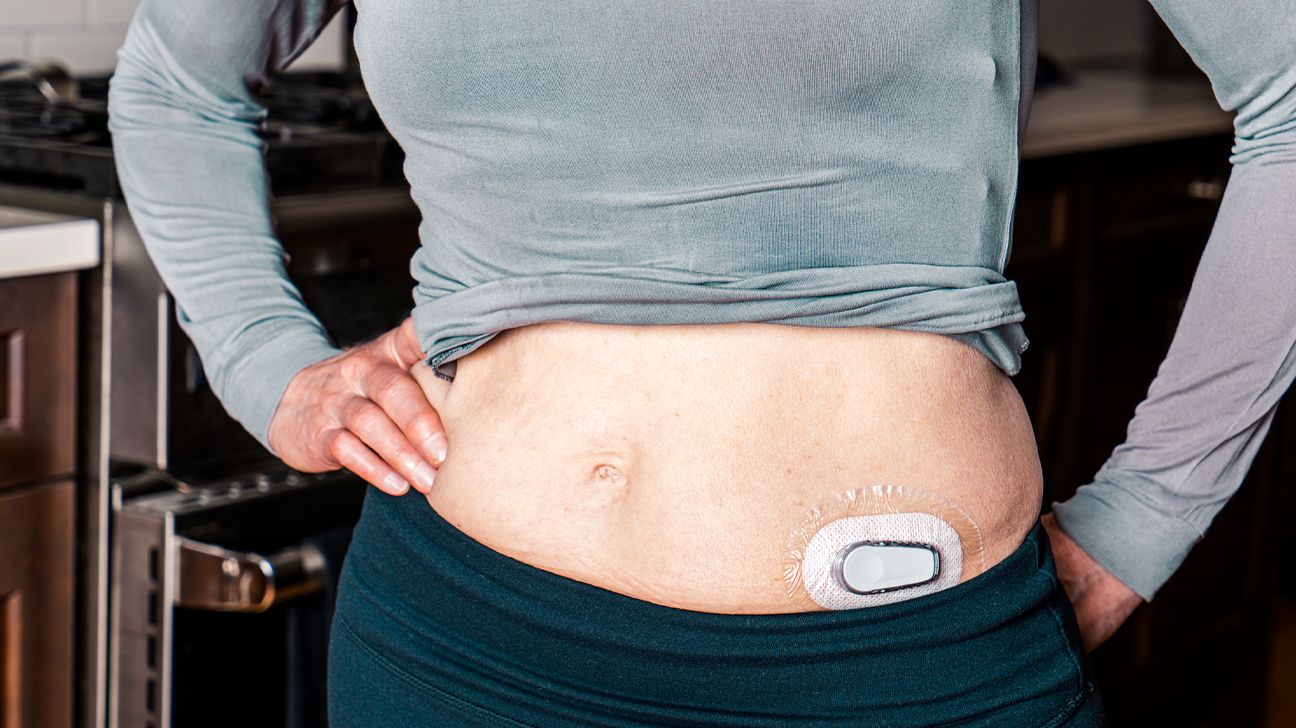
A continuous glucose monitor (CGM) is a compact medical device that continuously monitors your glucose levels, usually in 5-minute or less intervals, and streams that data to a smartphone app or another device for display.
Several companies make versions of a CGM. People with diabetes typically need a prescription for their monthly CGM supplies to manage their condition.
In recent years, non-prescription versions have also become available for those without the condition who might want to use the glucose-tracking technology to understand how food, exercise, or other factors affect their health.
This article will focus on CGM technology that is typically used by those with diabetes and typically requires a prescription.
To use a traditional CGM, you insert a small sensor into your abdomen or arm, and a tiny plastic tube known as a cannula penetrates the top layer of skin.
An adhesive patch holds the sensor in place, allowing it to take glucose readings in interstitial fluid (the fluid that surrounds cells in the body) throughout the day and night.
Generally, the sensors last 7 to 15 days. However, there is a newer implantable CGM that is different than traditional types of CGM and it lasts for up to a year.
Depending on the particular manufacturer and generation of CGM sensor, it would connect to a small reusable transmitter that is essentially the “brains” of the system.
The transmitter sends real-time readings to a smartphone app or another device (such as an insulin pump or a separate receiver) to display your blood glucose data. Some systems come with a dedicated monitor, and some now display the information via a smartphone app, so you don’t even need to carry an extra device around with you.
Aside from the constant stream of data, most CGMs can send alerts when your blood sugar levels are rising too high or dropping too low. You can also set personalized alerts for your particular glucose target range and customize how you’re notified.
It’s not an understatement to say that CGMs have revolutionized diabetes care.
Research shows that CGM technology is beneficial for all types of diabetes, including type 1 diabetes, type 2 diabetes, who may or may not be on insulin, and for those with gestational diabetes during pregnancy.
Traditionally, CGMs have been designed for people with type 1 diabetes. But in recent years, more people with type 2 diabetes have been using this technology and finding benefits compared to fingerstick management.
This has become a standard of care for the diabetes community.
Unlike a traditional fingerstick (blood glucose meters), which provides just a single glucose reading, CGMs provide continuous, dynamic glucose information every 5 minutes — roughly 288 readings in a day.
First off, you can literally see in real time the effects of food and exercise on your blood glucose levels, and catch cases of hyperglycemia (blood sugar too high) and hypoglycemia (blood sugar too low) as they happen, avoiding the potentially dangerous consequences.
This is a huge advantage over historic “static” blood glucose monitoring, which only provides a single glucose reading at a time.
Studies have shown CGMs to be among the best outpatient blood sugar management options for reducing A1C — the traditional “gold standard” test of blood glucose management.
Other
CGMs can be especially beneficial for active children (and adults, too), for ensuring safety during physical activity and during nighttime glucose fluctuations.
It’s also a potentially lifesaving tool for people with diabetes who experience hypoglycemia unawareness, alerting them to impending low blood sugars when their own bodies fail to recognize the warning signs.
Currently, there are 4 CGM systems approved by the Food and Drug Administration (FDA) and available in the United States.
Dexcom
San Diego-based Dexcom was the pioneer in this field, introducing the first-ever real-time CGM in 2006.
The latest version is the Dexcom G7, introduced in 2023.
An earlier model known as the Dexcom G6 CGM is still available, but many people with diabetes are being transitioned to the newer generation and the G6 is expected to be discontinued in 2026.
The G7 sensors are “factory calibrated,” eliminating the need for users to set baseline levels with a fingerstick test. They’re FDA-approved for safe use in children as young as 2 years old.
Many design aspects and features are similar between the G7 and G6.
- Wear-time: Each Dexcom sensor is labeled to be worn on the abdomen for up to 10 days before needing replacement. The company expects to launch a 15-day sensor in late 2025.
- Design: For the G6, a separate transmitter clicks into the plastic-base housing of each new G6 sensor. Each transmitter lasts for about 90 days before needing replacement. However, one big difference is that the G7 has a combined sensor-transmitter, compared to the G6, which has separate pieces you must buy and use for the CGM to work.
- Connectivity: The sensor-transmitter uses Bluetooth connectivity to talk with the Dexcom G6 mobile app for both iOS and Android devices, as well as Apple Watch and other devices, including insulin pumps like the Tandem t:slim X2 and Omnipod 5.
- Alerts and alarms: The system offers customizable alerts, compatibility with Dexcom’s Clarity software and smartphone app for reviewing data, and the ability to easily share device data with up to 10 followers (which can include your doctor, diabetes educator, caregiver, or family members). It also includes voice integration via Apple’s Siri technology.
Medtronic Minimed Guardian Connect
The longtime leader in insulin pumps also makes a CGM device called the Guardian. Originally, it was only sold in a combo system with its pumps. But in March 2018, the FDA approved Medtronic’s Guardian Connect, the company’s first stand-alone CGM in more than a decade.
This system includes a small sensor that can be worn on the upper arm or abdomen for up to 7 days and a Bluetooth transmitter that sends glucose readings to a cellphone app every 5 minutes.
Guardian’s smart technology predicts where glucose levels are headed and alerts users from 10 to 60 minutes before a “glucose excursion,” allowing them to take appropriate actions in advance to avoid high and low episodes.
The stand-alone Guardian Connect is FDA approved for users ages 14 to 75 years old, although the Guardian version connected with Medtronic’s Minimed 770G and 780G pumps is approved for use in younger children in the context of those combo systems.
Abbott FreeStyle Libre
Abbott has been a longtime diabetes technology manufacturer, but the company got into the CGM game in just the past decade with its unique FreeStyle Libre Flash glucose monitor. It’s been available overseas since 2014 and received FDA approval in 2017.
What’s different about a “flash” system is that users wear a small round sensor inserted on the upper arm, but it does not automatically send readings.
Instead, users must manually swipe the handheld receiver or smartphone app over the sensor to get glucose readings.
The latest version is the FreeStyle Libre 3, approved in 2022 for the United States.
The sensor is quite small—about the size of two stacked quarters—and can be scanned through clothing. It’s also water-resistant, allowing users to wear it when swimming or bathing. The sensor comes factory-calibrated, so it needs no calibration and is approved for 14-day wear.
Data can be read and analyzed on the receiver or a smartphone using Abbott’s LibreLink app. This app offers remote data sharing with up to 20 people. The Libre is FDA approved for ages 4 years and older.
Eversense implantable CGM
The Eversense system is the first long-term implantable CGM made by Senseonics.
It consists of a tiny sensor the size of a small twig implanted underneath the upper arm’s skin. Currently approved in the United States for up to a year of wear in the United States, it is by far the longest-lasting sensor.
The sensor must be inserted and removed by a doctor, who performs a small surgical incision under the skin at the clinic. A flat oval black transmitter is worn over the insertion site and held in place with an adhesive. The transmitter must be taken off and charged daily.
An iOS or Android smartphone app views and controls the system. The app also offers several data reports that can be easily sent to a doctor (or anyone) with the click of a button.
This CGM product is sold by Ascensia Diabetes Care, the same company that makes the Bayer Contour fingerstick meters.
As with most medical technology, you need a prescription to get a CGM for diabetes management. Your doctor can write a prescription for any of the above CGM devices.
But getting a prescription for a CGM is often not as simple as just walking into your doctor’s office and asking for one.
Instead, you’ll likely need a prior authorization (PA) to get a CGM through your health insurance. This is a form or process that your doctor has to go through to prove “medical necessity” to obtain approval from your insurance plan to cover the costs associated with the prescribed treatment (CGM, in this case).
At this point, most major insurers (and Medicare) require a PA before extending coverage for CGM.
Navigating insurance coverage of CGM technology
Here’s how to go about securing insurance coverage, according to Breakthrough T1D, a leading T1D research and support organization:
- Check your insurance plan’s policy documents and formulary to see if any of your treatments require a prior authorization. You may find these on the plan’s website. If you have Medicare coverage, check the Medicare & You handbook for more information.
- If a PA is needed, locate your insurance carrier’s submission process and obtain any required forms. This information is typically found on the plan’s website, or you may call the member services number on the back of your insurance card.
- Your doctor’s office is responsible for submitting PAs, so it is important to work with your doctor or the staff member designated to handle the paperwork.
- Ensure that the PA request is submitted according to the plan’s guidelines and double-check that you meet all requirements before they’re submitted.
- Once your request is submitted, the insurance company may approve or deny it. If approved, be aware that the approval letter may include rules about how you obtain the care. If so, you will need to abide by those terms to be covered.
- If the request is denied, you might consider appealing the decision.
You may be wondering what constitutes medical necessity? Here are some general PA criteria used to see if a patient meets requirements to get a CGM:
- Type 1 diabetes diagnosis
- completion of a comprehensive diabetes education program
- requires multiple, daily insulin injections or insulin pump therapy with frequent dosage adjustments
- documented average frequency of glucose self-testing greater than 4 times per day during the previous 2 months
- intention to use a monitoring device as an adjunct to standard care
- frequent unexplained hypoglycemia or hypoglycemic unawareness
CGMs are the most advanced tools currently available for diabetes care, but like everything else, they have pros and cons in terms of lifestyle impact.
Pros
- eliminate the need for fingerstick tests
- provide alerts and alarms for high and low glucose levels
- constant data stream can help you spot trends
- users report that they learn about their diabetes and its relationship to their bodies
Cons
- wearable tech — even without wires, you’ll still have to deal with having a device affixed to your body
- the constant data stream can be a drawback for some people, creating a sense of urgency to constantly react to the number you see on the screen
- if you’re very concerned about real-time safety alerts, you may want to skip the Abbott Libre system (at least until a new model comes out that includes alarms)
- costs are a major concern for many people
Here are some details that might help you determine which CGM is best for you:
- If you’re looking for long-standing reputation and the authority of market share, Dexcom fits that bill. The longest player in CGM technology, Dexcom generally boasts the most accurate readings of 8.2% of lab-measured glucose readings. With its G7 model, Dexcom offers high and low alerts, customizable alarms, data sharing, and a water-resistant device.
- Medtronic’s Guardian Connect is the most similar to Dexcom’s line, and the costs are on par. The Guardian Connect is reportedly close in accuracy (within 10% of lab values), although many patients claim that Dexcom seems more accurate in real-world settings.
- The Guardian Connect offers increased connectivity, with data automatically uploaded into the company’s Carelink app. That means your doctors can get your numbers without you having to do a thing. The predictive alerts are an interesting feature as well.
- Abbott’s FreeStyle Libre emphasizes cost-effectiveness and streamlined design. However, some models don’t offer the features traditional CGMs provide, including true continuous monitoring and programmable high-low alerts.
- The implantable Eversense offers some cutting-edge features but has some potential drawbacks. On the upside, the system is very accurate, has the longest sensor life, and offers customizable alerts. Even though it’s implantable, you must always wear a small black unit attached to your upper arm to get readings.
- With Eversense, you must also have it inserted and removed by a medical professional. This currently means visiting your doctor’s office every three months to have the sensor removed and a new sensor inserted. Some people have reported small scars on their shoulders as a result.
CGM technology has been a game-changer for people with diabetes, as it offers a hugely improved ability to constantly track glucose levels.
Unlike traditional fingerstick meters, which only provide a snapshot of blood sugars at the moment, CGM systems provide a complete picture of how one’s blood sugars are trending both in the moment and over time.
Many CGMs are being connected to insulin delivery devices to create combo systems that can automatically adjust insulin doses based on the glucose data being streamed via Bluetooth.




