Deep brain stimulation (DBS) is a treatment for various neurological conditions such as Parkinson’s disease and obsessive-compulsive disorder. It involves surgically implanting an electrode into your brain.
Research on using electricity to treat neurological conditions has been ongoing since at least the
Modern DBS was first approved by the Food and Drug Administration (FDA) in 1997 for essential and Parkinsonian tremors, which are caused by Parkinson’s disease and related conditions.
Electrical impulses from a DBS electrode may help modify your brain activity to reduce certain activities.
DBS isn’t a cure for neurological conditions, but it can significantly help reduce symptoms and improve quality of life for many people.
Learn more about DBS, including how it works, what to expect during the procedure, and potential side effects.
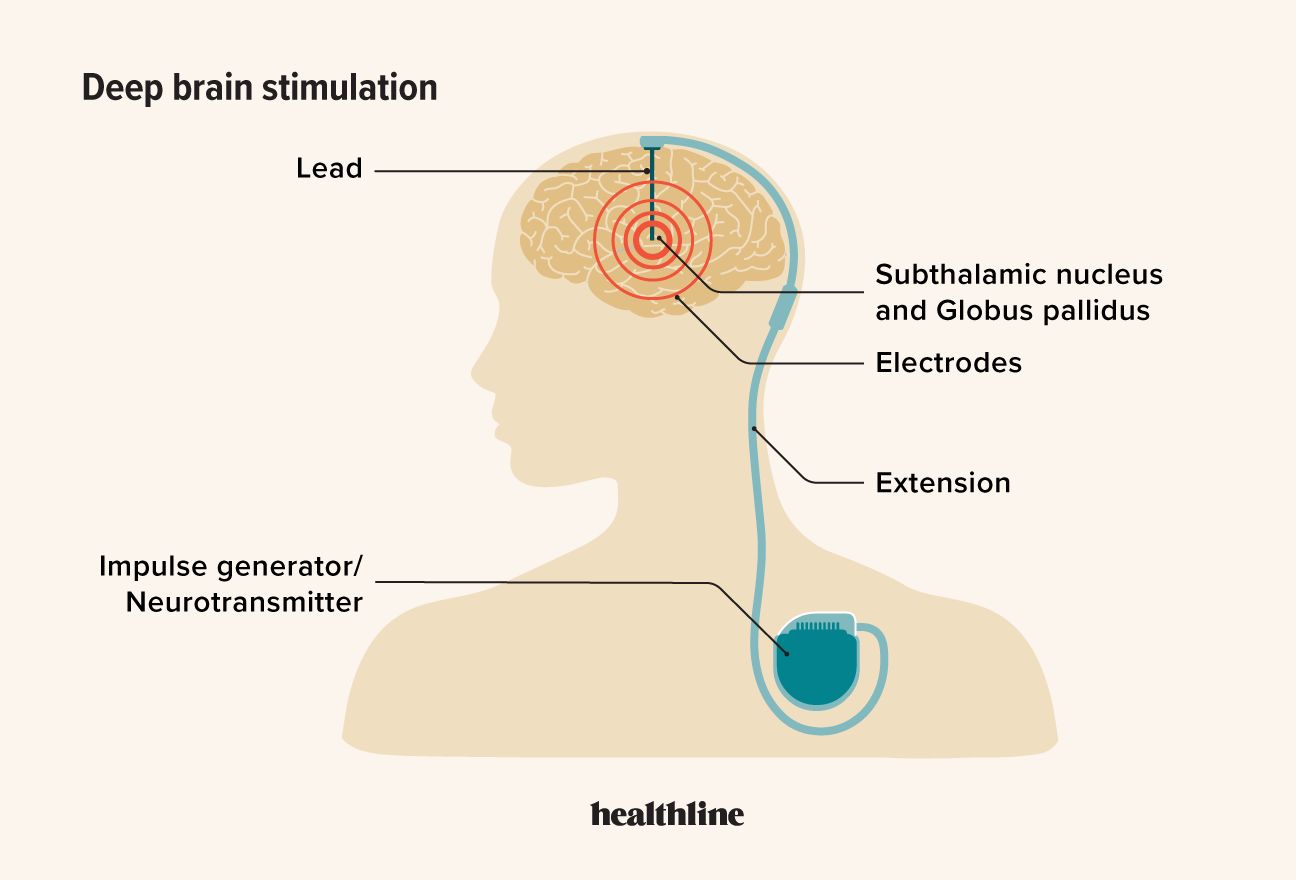
DBS works by delivering precise electrical pulses from an electrode to specific parts of your brain. This electrical activity may modify your brain’s electrical signals to help improve symptoms of certain neurological conditions.
The components of a DBS system include:
- Pulse generator (neurostimulator): A battery-powered device implanted under your skin, usually near your collarbone.
- Leads (electrodes): Thin wires implanted in specific areas of your brain that deliver electrical stimulation.
- Extension wires: Insulated wires that connect the electrodes in your brain to the pulse generator.
- External programmer: A device used by healthcare professionals to adjust the settings of the neurostimulator without the need for surgery.
DBS is usually reserved for people with certain neurological conditions that don’t respond well to medication or other less invasive procedures. Here are some of the conditions it’s used to treat.
Parkinson’s disease
DBS was first approved to treat Parkinson’s disease in 2002. It’s mostly used when medications aren’t able to fully help a person with advanced disease regulate their symptoms.
DBS could help improve tremor and movement for some people, though it doesn’t stop disease progression. DBS is also used to treat tremors caused by Parkinsonian disorders, which are a group of conditions related to Parkinson’s.
Dystonia
DBS has been FDA-approved to treat dystonia since
People with dystonia experience involuntary muscle contractions that often cause twisting and abnormal postures. DBS may help reduce muscle contractions and improve mobility for some people, particularly in those who don’t respond to medication.
Essential tremor
DBS was first FDA-approved to treat essential tremor in 1997. Stimulation of specific brain areas can potentially lessen tremors and enable better daily functioning.
Epilepsy
DBS was FDA-approved in
Obsessive-compulsive disorder (OCD)
DBS was FDA-approved to help treat OCD in 2009. Like with other conditions DBS is approved to treat, DBS for OCD is usually reserved for severe cases that don’t respond well to less invasive treatments like medications.
Other conditions
Research is ongoing on whether DBS may be able to help treat other neurological and psychiatric conditions. Some of the conditions
While results are promising in some cases, these uses are considered experimental, and more research is needed.
Here’s what you can expect before and during DBS.
Preparation
Before surgery, you’ll undergo a thorough evaluation. This usually includes consultation with neurologists and neurosurgeons to see if DBS is appropriate.
You’ll also likely receive an MRI scan so that your surgeon can see the area that needs to be treated. If you have a tremor, involuntary movements, or anxiety about the scan, a doctor will likely prescribe a sedative because you need to remain still during an MRI for the image to be processed clearly.
Rarely, people with severe involuntary movements may need general anesthesia to put them asleep during the imaging.
DBS procedure
DBS surgery is typically performed in two procedures, which are usually done on the same day.
During the first, you’ll likely:
- Have your head shaved, or at least the part around your surgical area.
- Your head will be placed in a special frame to keep your head still.
- You’ll likely receive a local anesthetic during the procedure to numb the area. But you’ll probably remain awake in order to provide feedback to the surgeon.
- A CT scan will be used to find the correct place to make an incision.
- Your surgeon will make a small incision and drill a hole in your skull so they can implant the device.
- They may ask you questions during the procedure or move your limbs in certain ways.
Depending on the condition, the second procedure can be done while you’re awake or asleep. For epilepsy, you’ll usually be given a general anesthesia to put you asleep.
You’ll also be given a sedative to relax during the surgery.
During the second procedure:
- Your surgeon will make an incision below your collarbone and create a space to hold the pulse generator.
- They’ll then insert the extension wires that travel between your skull and the underside of your skin.
- They’ll connect the parts of the device together and close your wounds.
- You’ll likely be able to go home the same day as your surgery.
After surgery, the system will be activated and programmed over several weeks. Adjustments are made to optimize symptom relief and minimize side effects.
When the DBS device is first activated, you may experience a range of sensations, from immediate improvement in symptoms to mild tingling, tightness, or other unusual feelings. These effects are monitored and settings adjusted to maximize benefit and minimize discomfort.
Potential side effects you’ll experience when your device is turned on include:
- tingling or numbness
- muscle contractions or spasms
- speech or vision changes
- mood alterations, including depression or euphoria
- difficulty with balance or coordination
- reversible side effects, such as slurred speech or tingling when the device is turned on
Most side effects are temporary and improve with adjustments to the stimulation settings.
DBS is generally safe, but as with any surgical procedure, there are risks.
According to a 2022 study in 426 people who had DBS surgery, the most common complication was infection, which affected 12 people (2.8%). This may require the removal of the DBS system or revision surgery.
The most serious complication is a stroke, according to the United Kingdom’s National Health Service (NHS). This occurs in about 1 in 100 surgeries. About half of these people make a recovery within 3 months. There’s a small risk of death, which occurs in about 1 in 500 surgeries.
Other potential complications include:
- provoked seizures
- confusion or changes in your behavior
- breakage of your device
Your recovery timeline depends on factors such as what condition you’re having treatment for and your overall health
Many people leave the hospital on the day of their procedure. Full recovery may take several weeks, with gradual improvement in symptoms as the device is programmed and adjusted.
DBS can be a very expensive procedure, often costing tens of thousands of dollars, depending on factors such as geographic area and the hospital where you have your surgery.
In a 2024 study, researchers found that the average cost in the United States using data from 26 articles from 2001 to 2021 was around $40,942.85, and the total cost after 1 year was $47,632.27.
The price includes pre-surgical evaluations, surgery, device, follow-up visits, and ongoing maintenance.
Is DBS covered by insurance?
DBS is typically covered by Medicare and most private insurance plans for FDA-approved conditions such as Parkinson’s disease, essential tremor, dystonia, and epilepsy.
Coverage for other uses may require special authorization for coverage.
Learn more: Medicare for DBS
DBS is a potential treatment for many neurological conditions. It’s generally reserved for cases that don’t respond to other, less invasive procedures.
DBS improves symptoms for some people with conditions such as essential tremor or Parkinson’s disease. It’s generally safe, but like all procedures, there’s a small risk of serious complications.




